We are a "Validation Engineering Operation" that seeks to optimize medical supply manufacturing facilities. Making use of the knowhow and results developed from special facilities management, we service air conditioning, utility, and other areas from a single validation list.
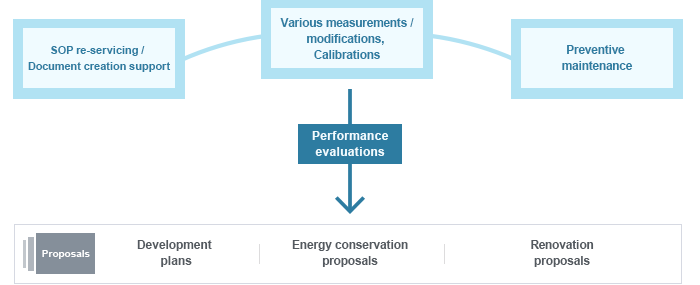
For the high level of sterility demanded of cleanroom environments, regular evaluations (annual reviews) are imperative.
We provide our own measurement technicians who expertly conduct cleanroom performance evaluation and diagnosis. We carry out our measuring services while focusing on established standard items of Operational Qualification (OQ) assessments. In order to verify cleanroom performance, those standard items and the measuring order is set in advance.
|

- Loop calibration
Inspect the precision and tolerance of the entire control system
- Simple Calibration
Determine the precision and tolerance of the detector alone

- We leverage the strengths of a wide range of independent vendors irrespective of the companies.
- We conduct preventative maintenance checks and produce GMP-compliant documentation according to our test specifications.
Nippon Air Conditioning Services validation support offers comprehensive services relating to air conditioning validation.
|
Client consultation |
|
Management plan creation support |
|
SOP revision support |
|
Fixed-term validation service (air conditioning facilities) |
|
Document preparation |
|
Measurement analysis, improvement proposals |